Objective
to compare whether 3/6-month DAPT was non-inferior to 12-month DAPT after implantation of DES with ultrathin struts and advanced polymer technology
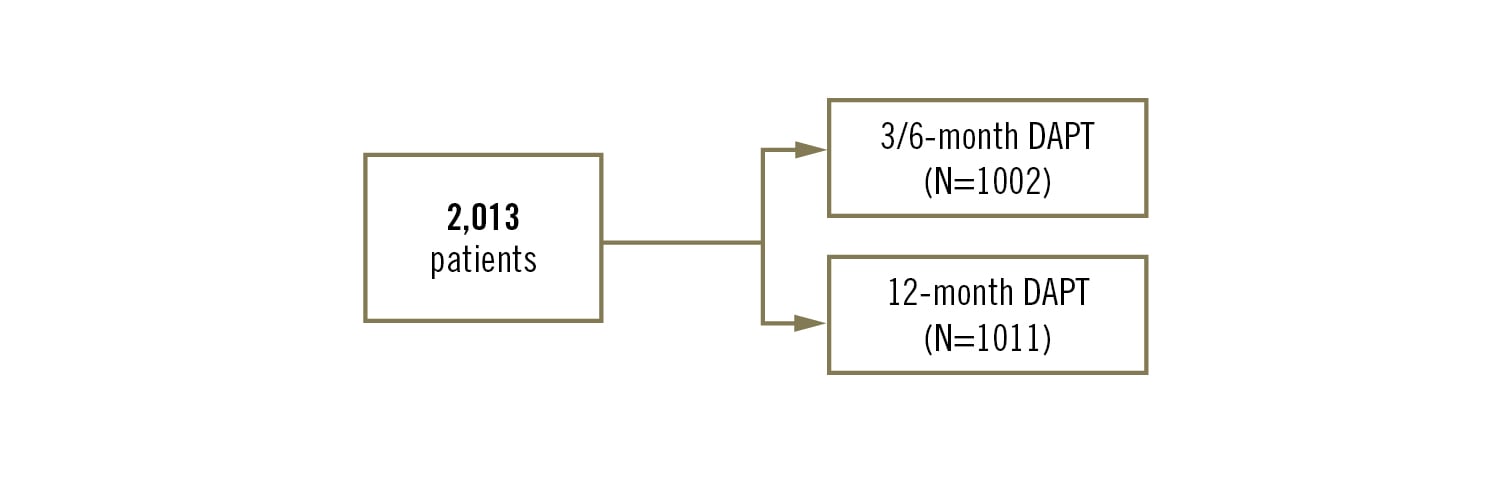
Study
prospective, multicentre, open label, randomised clinical trial
Population
adults with ischaemic heart disease (CCS or NSTE-ACS) undergoing PCI with the Orsiro biodegradable polymer DES or Coroflex ISAR polymer free DES
Endpoints
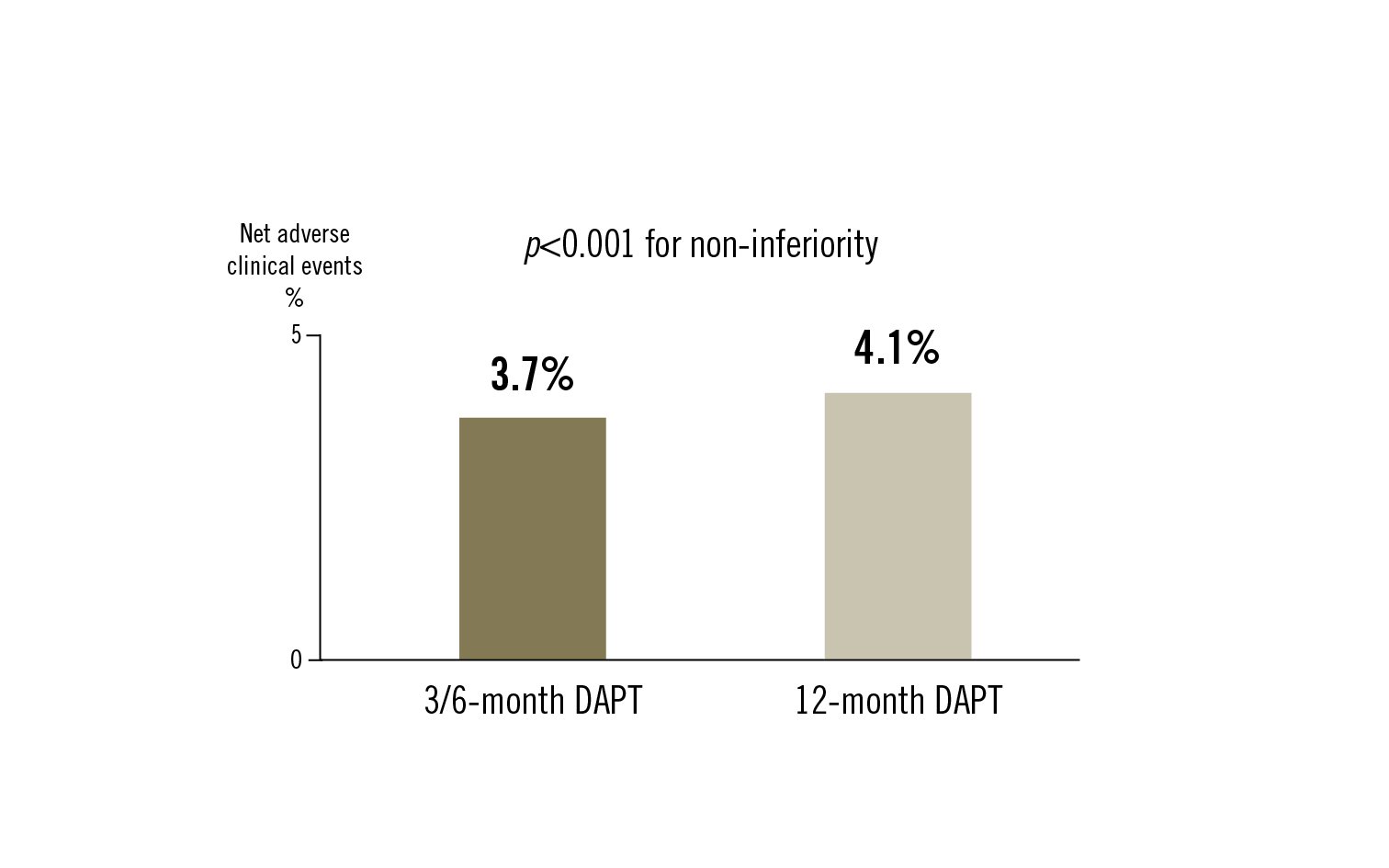
net adverse clinical events: a composite of cardiac death, target vessel MI, clinically driven TLR, ST or BARC types 3 or 5 bleeding at 12 months
Conclusion
among patients undergoing PCI using third-generation DES, 3/6-month DAPT was non-inferior to 12-month DAPT for net adverse clinical events
Han et al Circulation. 2023