Objective
to report the 5-year clinical outcomes of bioresorbable polymer SES (BP-SES; Ultimaster) compared to permanent polymer EES (PP-EES; Xience)
Study
prospective, single-blind multicentre, non-inferiority randomised trial
Population
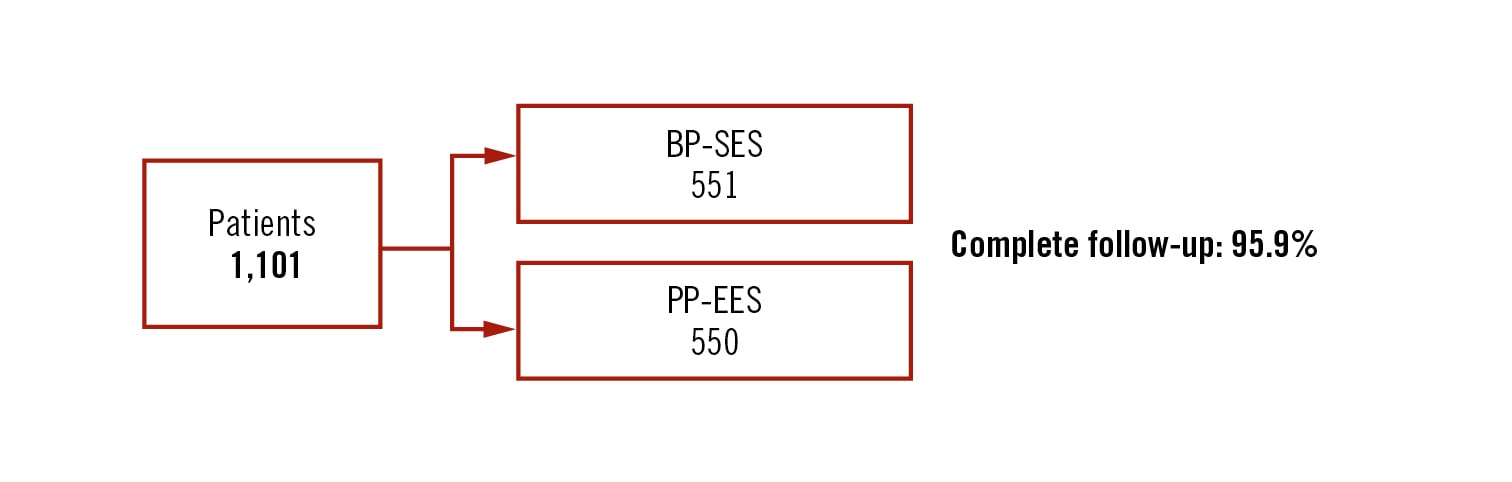
all-comers
Endpoints
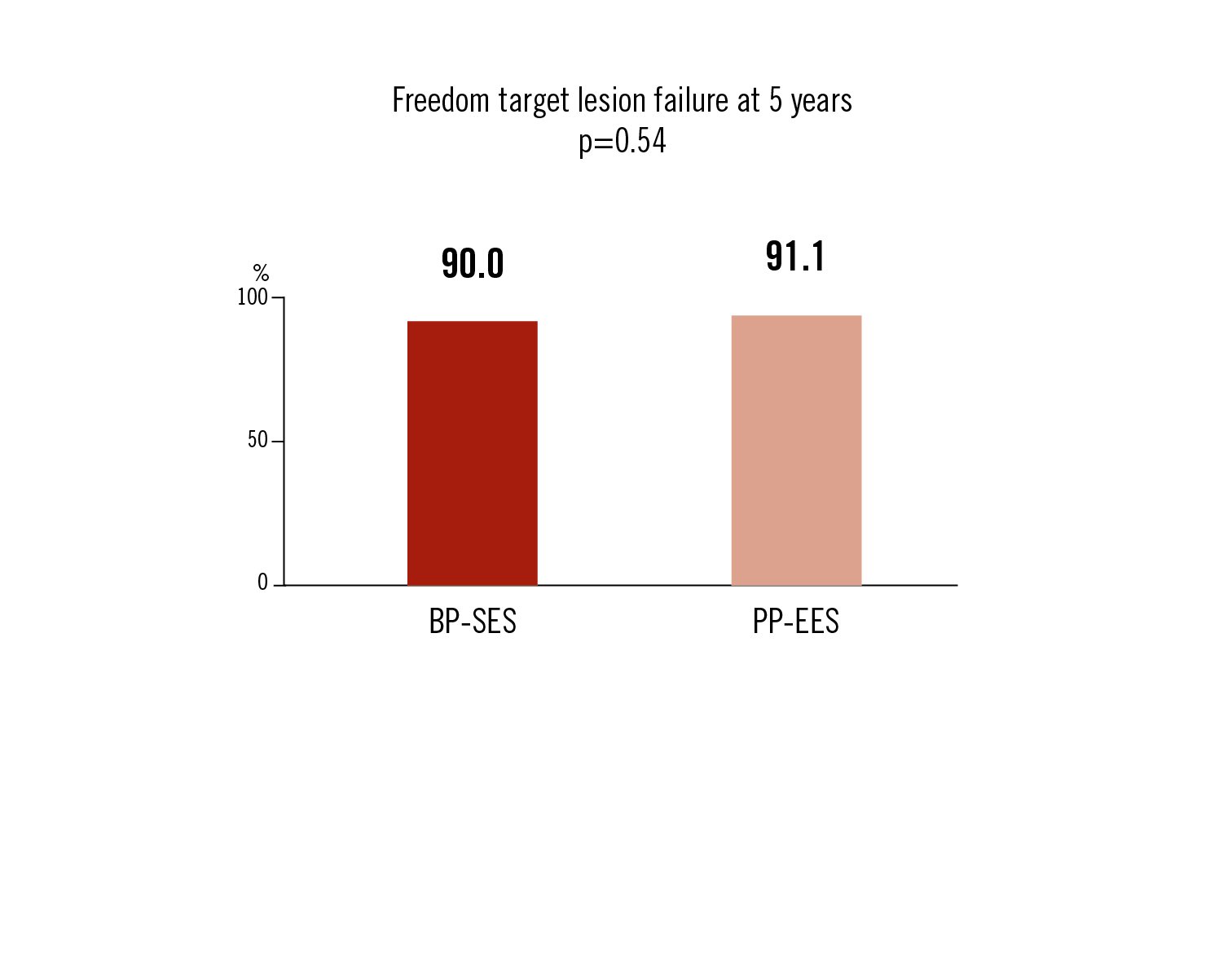
freedom of target lesion failure: cardiac death, TU-MI and target lesion revascularisation at 5 years
Conclusion
the BP-SES stent was non-inferior to PP-EES stent in terms of freedom of target lesion failure at 5 years
Wijns et al. EuroIntervention. 2018;14:e343-51