Objective
to compare angioplasty with nitinol constrained paclitaxel-coated Chocolate Touch DCB vs Lutonix paclitaxel-coated DCB for the treatment of symptomatic obstructive femoropopliteal disease
Study
prospective, multi-centre randomised controlled trial
Population
patients with claudication or ischemic rest pain (Rutherford class 2-4) and superficial femoral or popliteal disease (â¥70% stenosis)
Endpoints
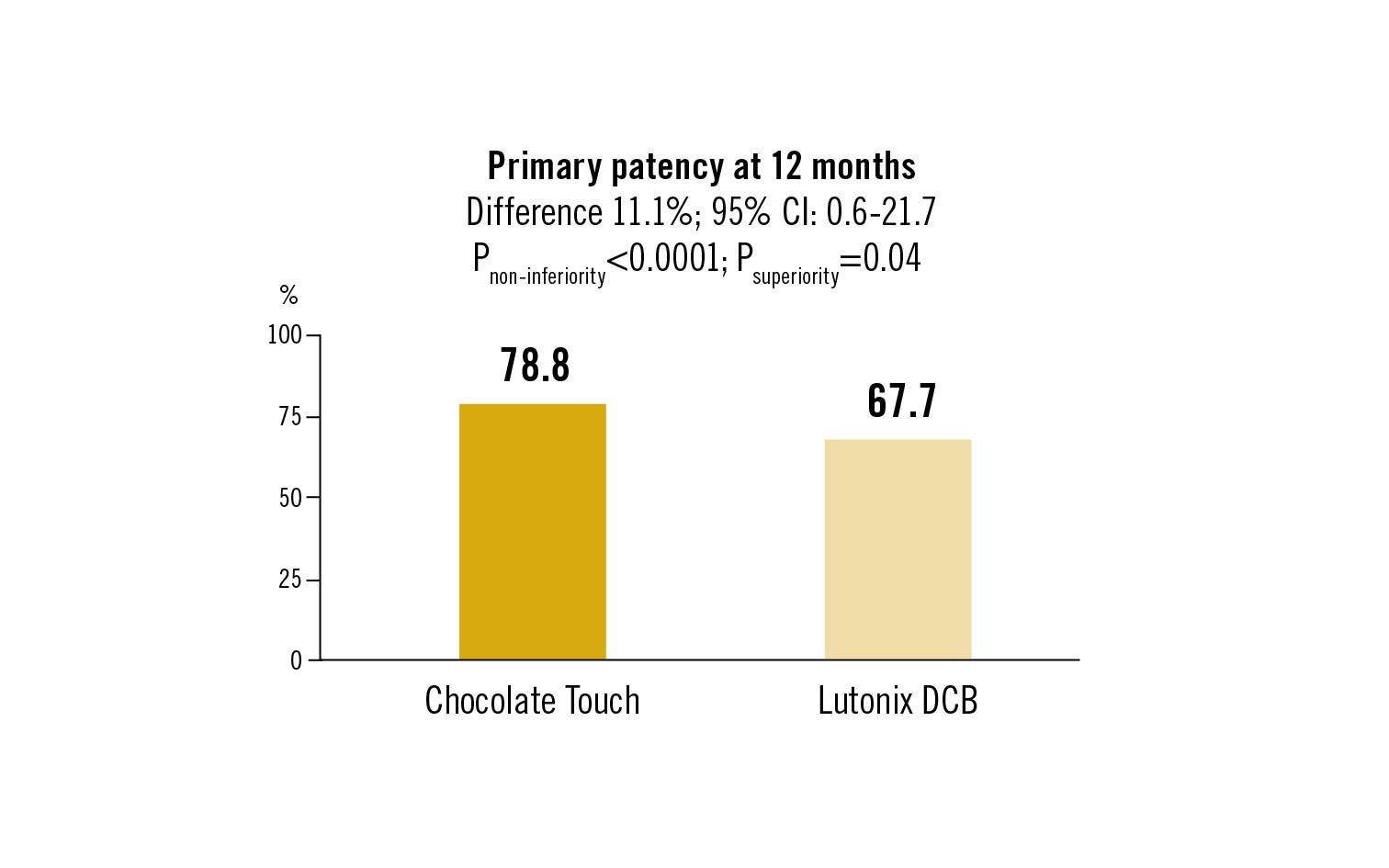
primary patency at 12 months; freedom from major adverse events at 12 months (peak systolic velocity ratio <2.4 without clinically driven TLR in the absence of bailout stenting)
Conclusion
for the treatment of femoropopliteal disease, Chocolate Touch DCB met both non-inferiority endpoints for efficacy and safety and was more effective than Lutonix DCB at 12 months
Shishehbor et al. Circulation. 2022; 145:1645-54