Objective
to compare the 1-year clinical outcome of polymer-free biolimus-coated Biofreedom stent with the ultra thin biodegradable polymer sirolimus-eluting ORSIRO stent
Study
registry-based, multicentre, single-blind non-inferiority randomised trial (non- inferiority margin 0.021)
Population
all-comers (53% ACS)
Endpoints
MACE: cardiac death, target vessel MI or target lesion revascularisation within 1 year

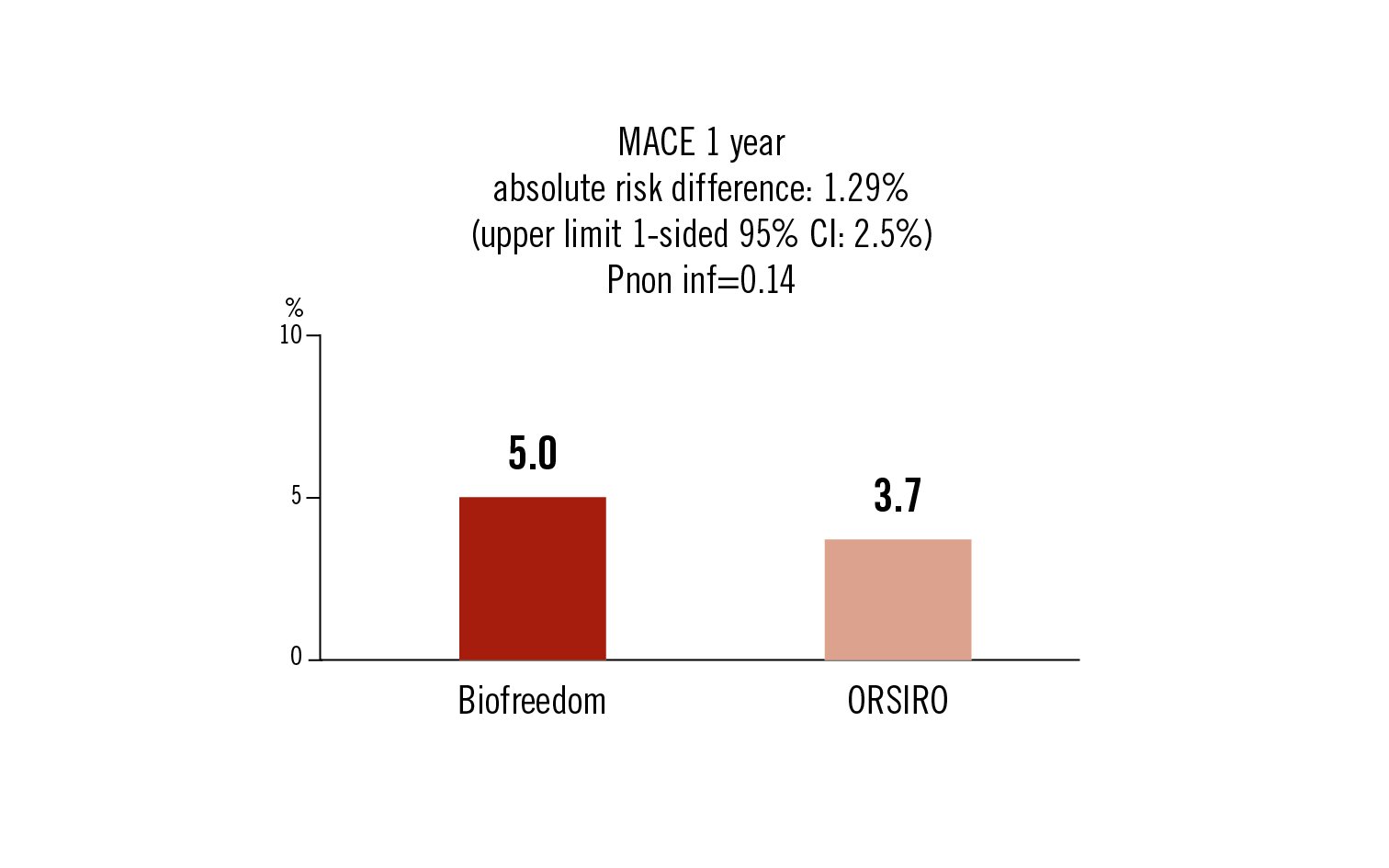
Conclusion
the Biofreedom stent did not meet criteria for non inferiority for MACE at 12months when compared to the ORSIRO stent
Okkels-Jensen et al. Circulation. 2020;141:2052-63