Objective
to investigate the clinical outcomes of drug-coated balloons compared to second generation DES (Xience or paclitaxel-eluting Taxus element stent)
Study
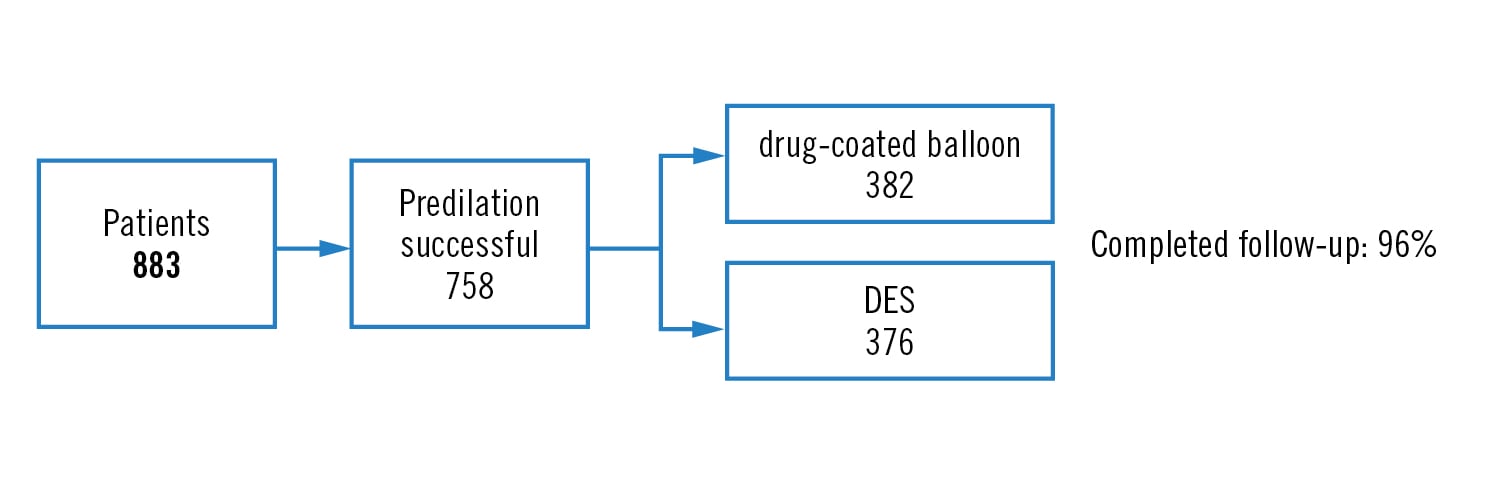
prospective, open-label, multicenter randomised non-inferiority trial (margin 4.0%)
Population
all-comers with de novo lesion in arteries <3Â mm
Endpoints
MACE as composite of cardiac death, non-fatal MI and TVR at 12 months
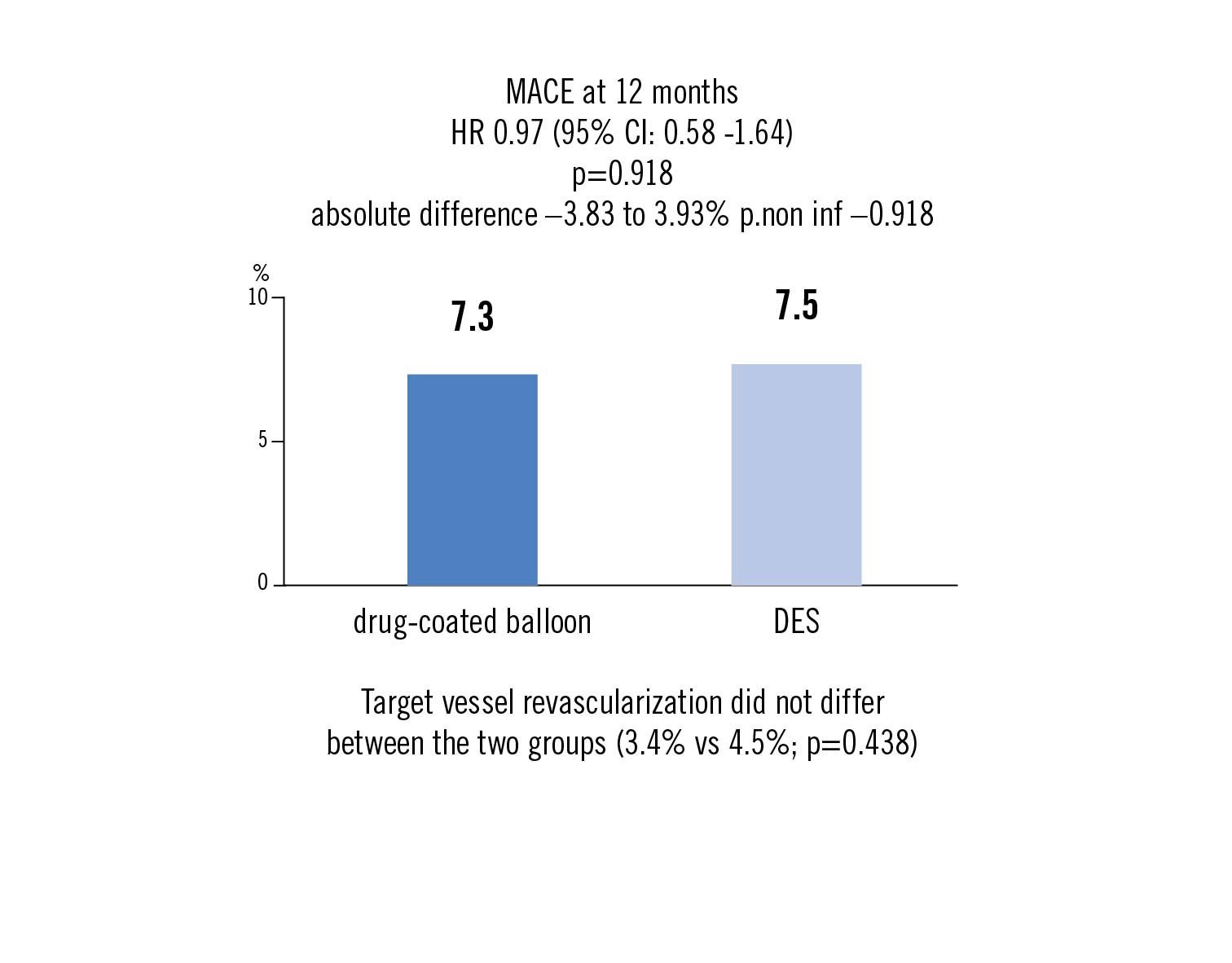
Conclusion
the 12 month adverse event rate of drug-coated balloons is non-inferior to DES for treatment of coronary artery lesions in small vessels in all-comers
Jeger et al. Lancet. 2018;392:849-56