Objective
to compare edoxaban with Vitamin K antagonists in patients with prevalent or incidental atrial fibrillation as the indication for oral anticoagulation after TAVI
Study
multicentre, randomised, open-label clinical trial
Population
post TAVI patients
Endpoints
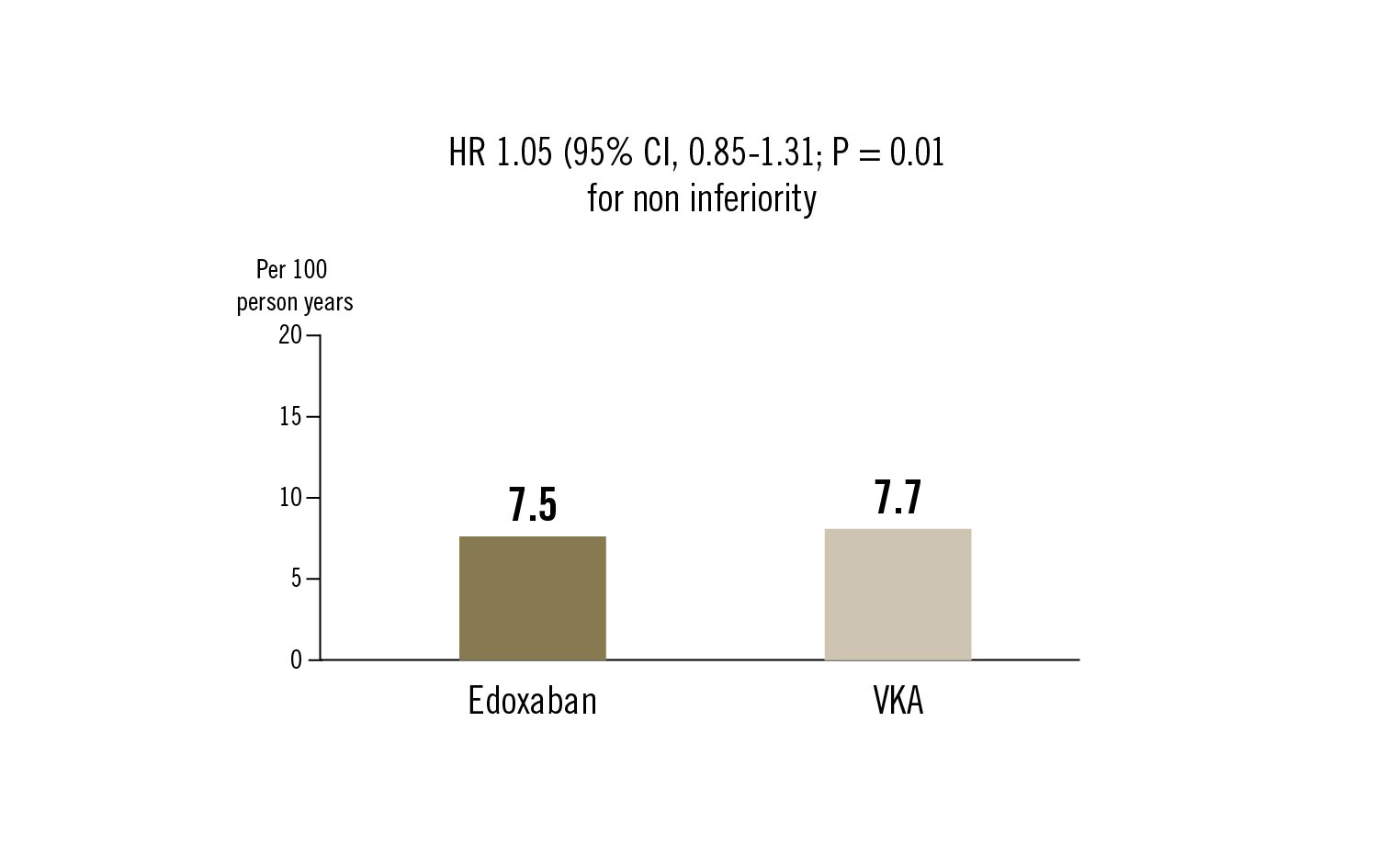
primary efficacy outcome was a composite of adverse events consisting of death from any cause, myocardial infarction, ischaemic stroke, systemic thromboembolism, valve thrombosis or major bleeding. The primary safety outcome was major bleeding
Conclusion
in patients with atrial fibrillation who underwent successful TAVI, edoxaban was non inferior to vitamin K antagonists for a composite primary outcome of adverse clinical events. The incidence of major bleeding was higher with edoxaban than with vitamin K antagonists
Van Mieghem et al. N Engl J Med. 2021