Objective
to compare the clinical efficacy of a thin-strut biodegradable polymer everolimus-eluting platinum-chromium stent (EES) with a bio-degradable-polymer biolimus-eluting stainless-steel stent (SES)
Study
multicentre randomised non-inferiority trial (margin of 3% risk at 12 months)
Population
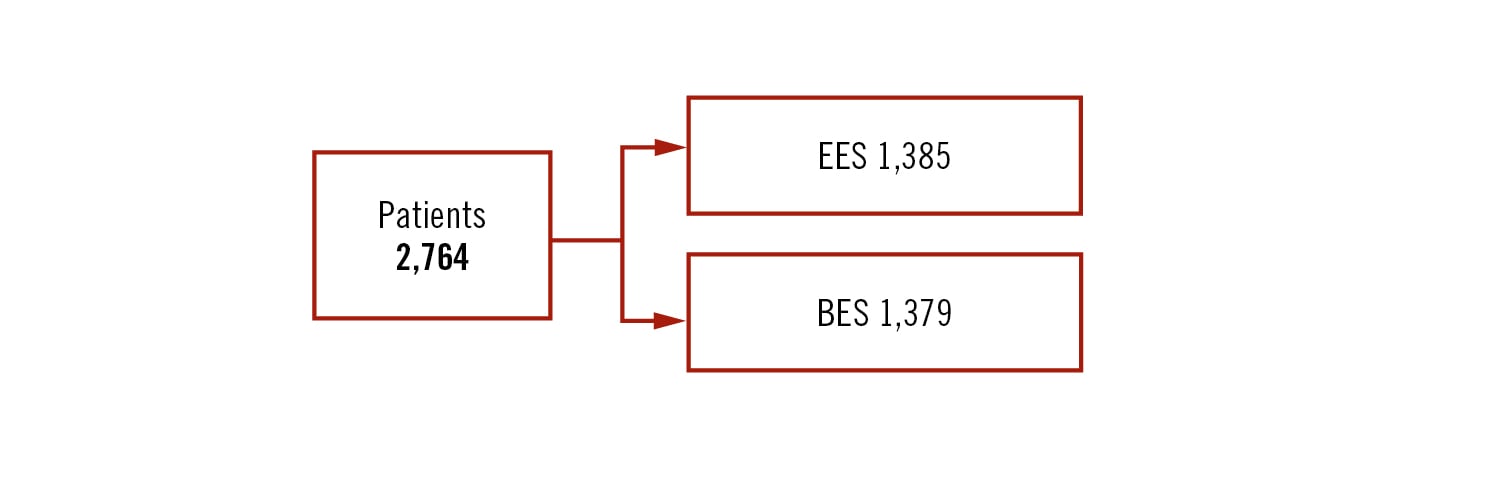
all-comers
Endpoints
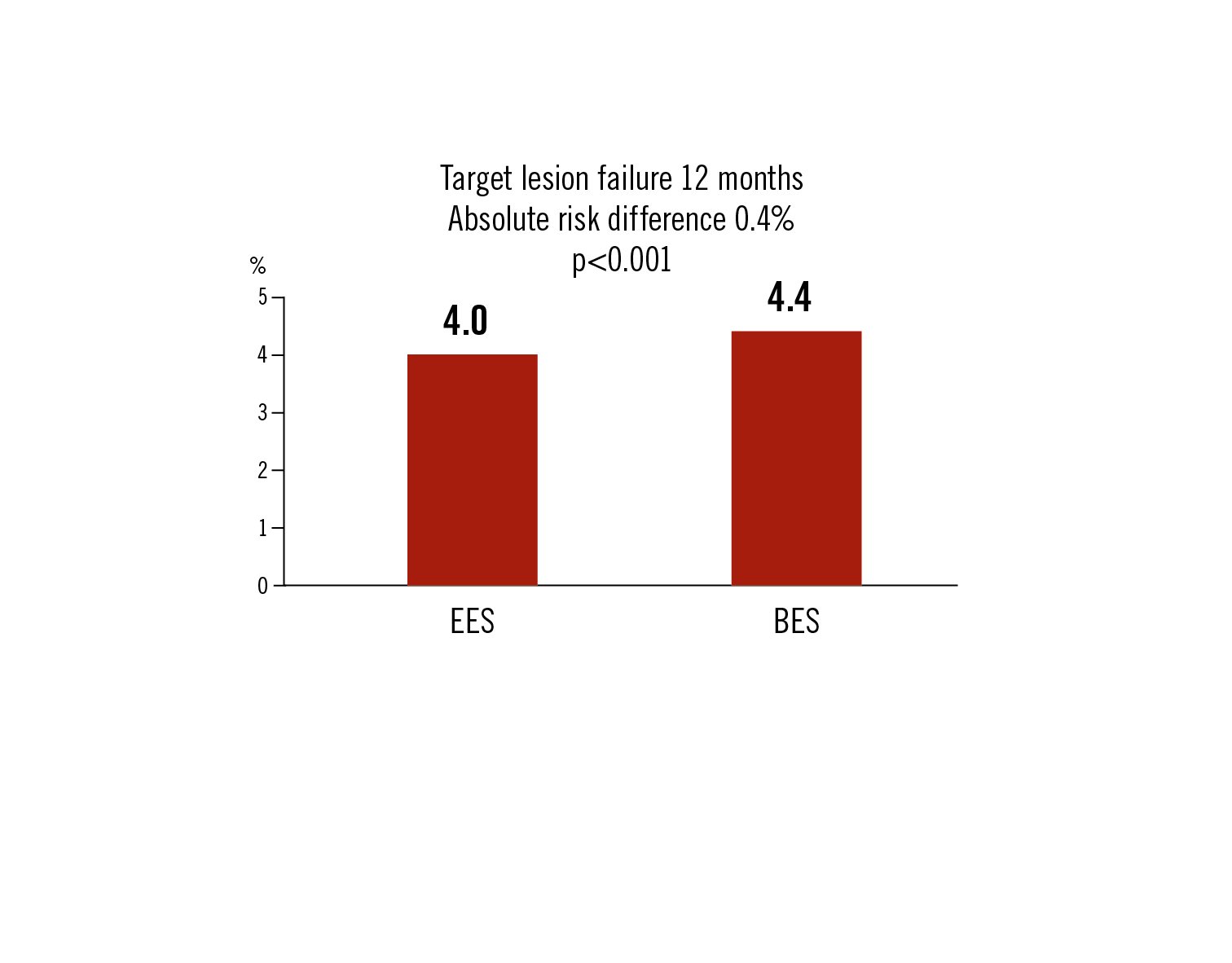
target lesion failure as a composite of safety (cardiac death and MI) and efficacy (target lesion revascularisation) at 12 months
Conclusion
EES was non-inferior to BES in terms of major adverse events at 1 year
Maeng et al. J Am Coll Cardiol Intv. 2019;12:624-33