Objective
to investigate the clinical outcomes of a localised abluminal groove low-dose sirolimus-eluting biodegradable polymer coronary stent (Firehawk) compared to a durable polymer everolimus-eluting stent (Xience)
Study
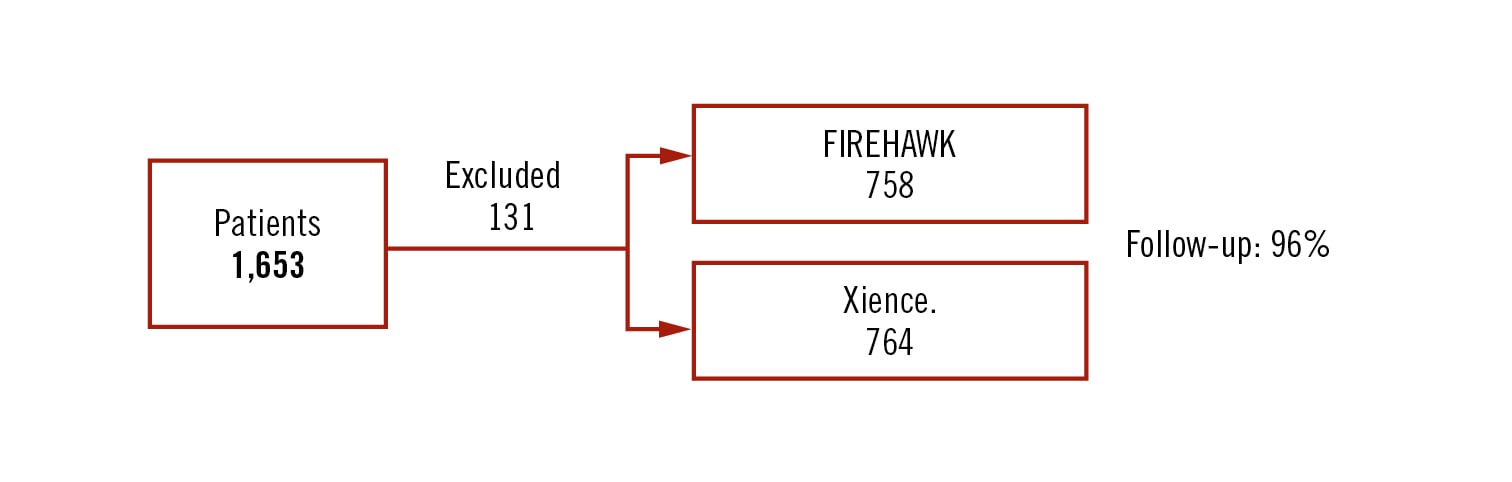
prospective, multicentre open-label non-inferior randomized trial (non-inferiority margin 3.5%)
Population
all-comers (stable angina 47%)
Endpoints
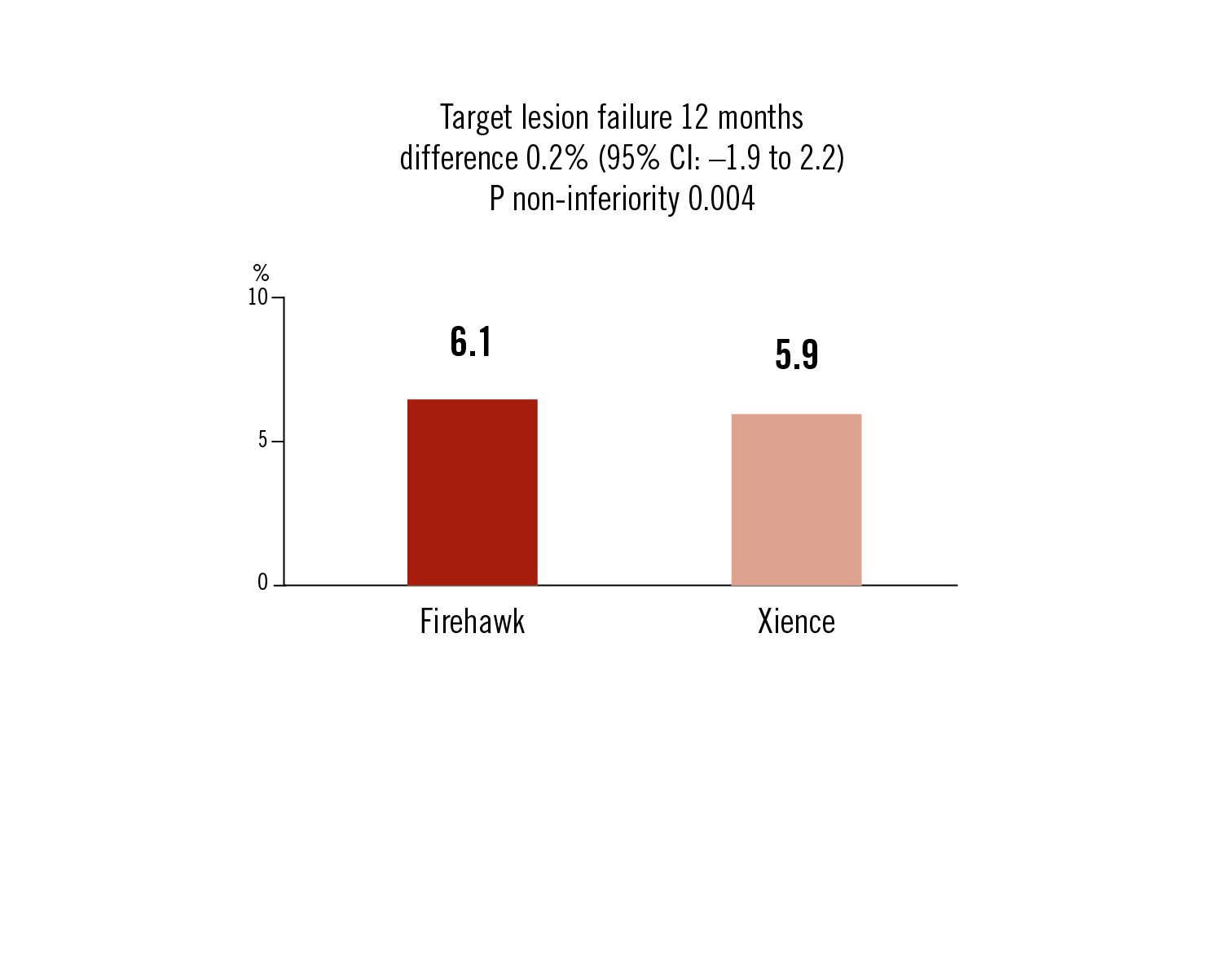
target lesion failure: cardiovascular death, target-vessel MI and ischemia-driven target lesion revascularisation at 12 months
Conclusion
target lesion failure: cardiovascular death, target-vessel MI and ischemia-driven target lesion revascularisation at 12 months
Lansky et al. Lancet. 2018;392:1117-26