Objective
to investigate whether half-dose Tenecteplase and PCI if indicated is as effective as primary PCI in older patients with STEMI
Study
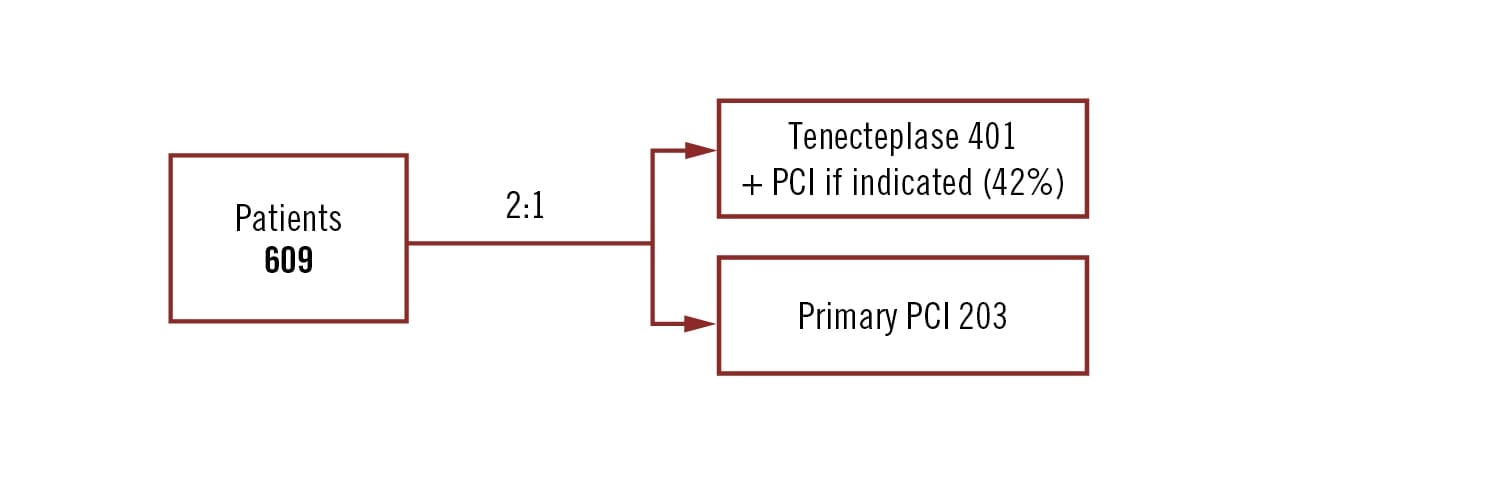
investigation-initiated, open-label multicentre, randomised trial
Population
patients ⥠60 yrs, presenting within 3 hours of symptom onset and unable to undergo primary PCI within 1 hour
Endpoints
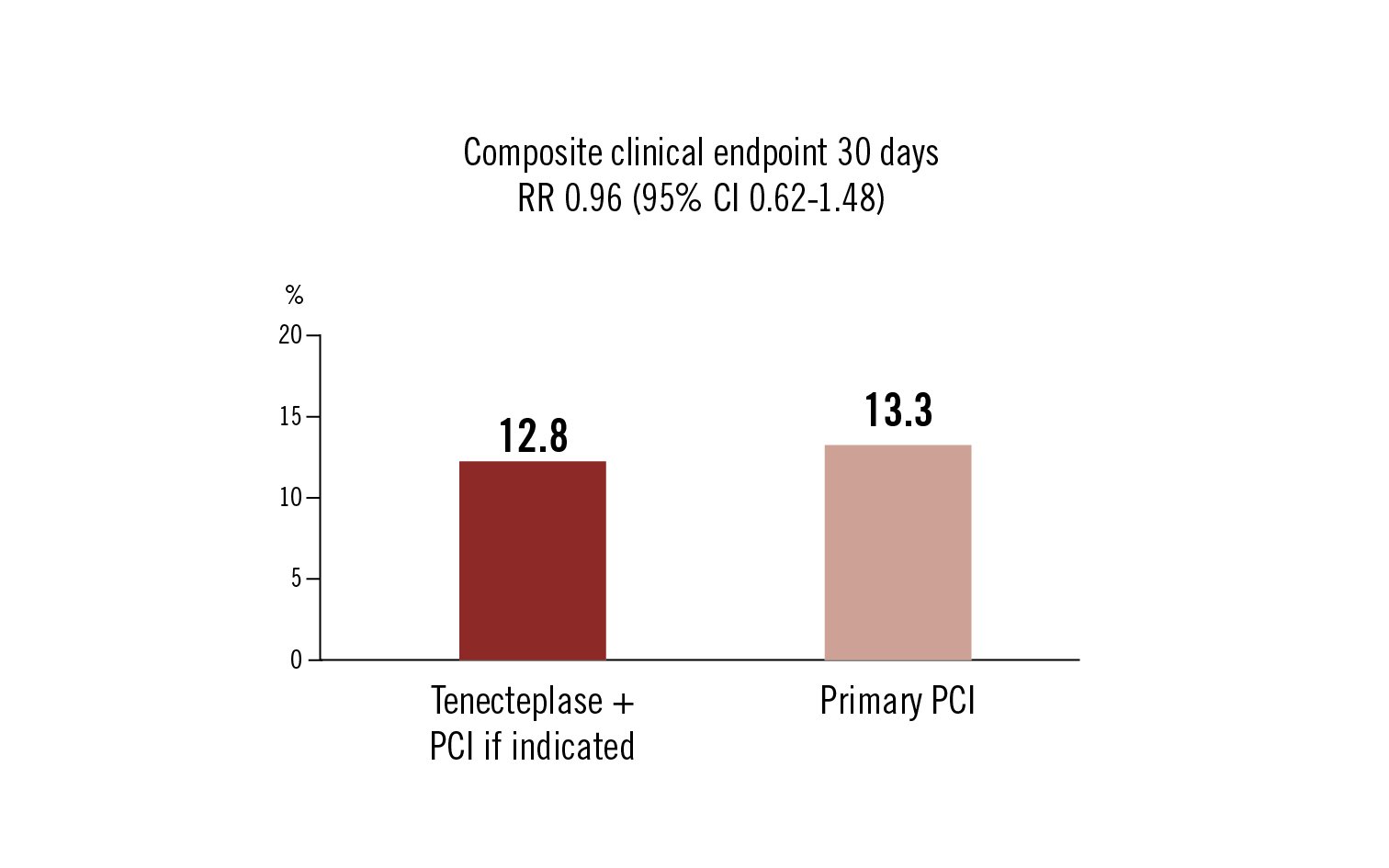
composite all-cause death, shock, heart failure and re-MI at 30 days
Conclusion
the incidence of adverse clinical events at 30 days was similar amongst older patients with STEMI treated with half-dose Tenecteplase (+PCI if indicated) as compared to primary PCI
van der Werf et al. Circulation 2023: 148; 753-764