Objective
to compare the 1-year safety and efficacy of the Passeo-18 Lux drug-coated balloon (DCB) with In.Pact Admiral DCB for the treatment of symptomatic peripheral artery disease (PAD)
Study
prospective multicentre non-inferiority randomised trial (margin 10%)
Population
patients with PAD caused by stenosis, restenosis or occlusion of femoral and/or popliteal arteries
Endpoints
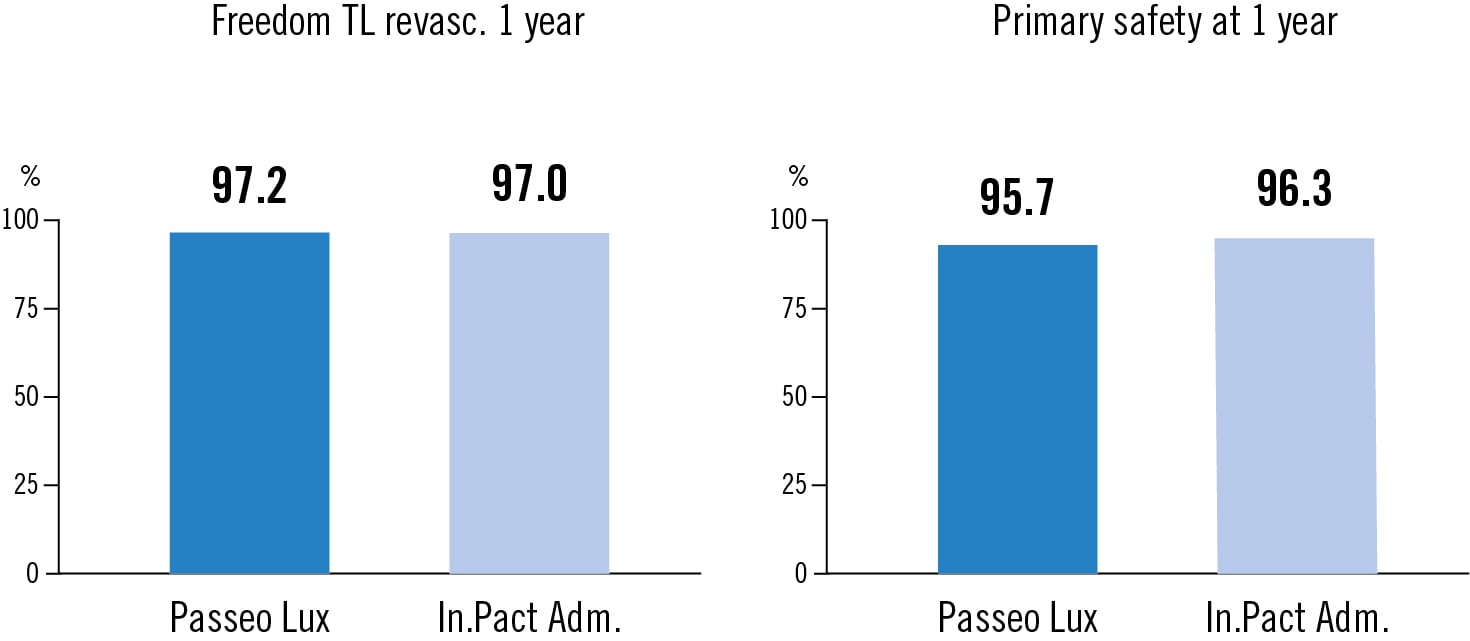
efficacy: freedom from clinically driven target lesion revascularisation at 1 year Safety: freedom of death, limb amputation or target vessel revascularisation at 1 year
Conclusion
The Passeo Lux DCB and In.Pact Admiral DCB demonstrate comparable efficacy and safety outcomes at 1 year follow-up
De Hoose et al. J Am Coll Cardiol Intv. 2023;16:2900-2914