Objective
to assess the clinical outcomes of a polymer-free amphilimus-eluting stent (AES) compared with a biodegradable polymer-everolimus-eluting stent (EES) for PCI treatment of CAD
Study
investigator-initiated non-inferiority trial
Population
all-comers
Endpoints
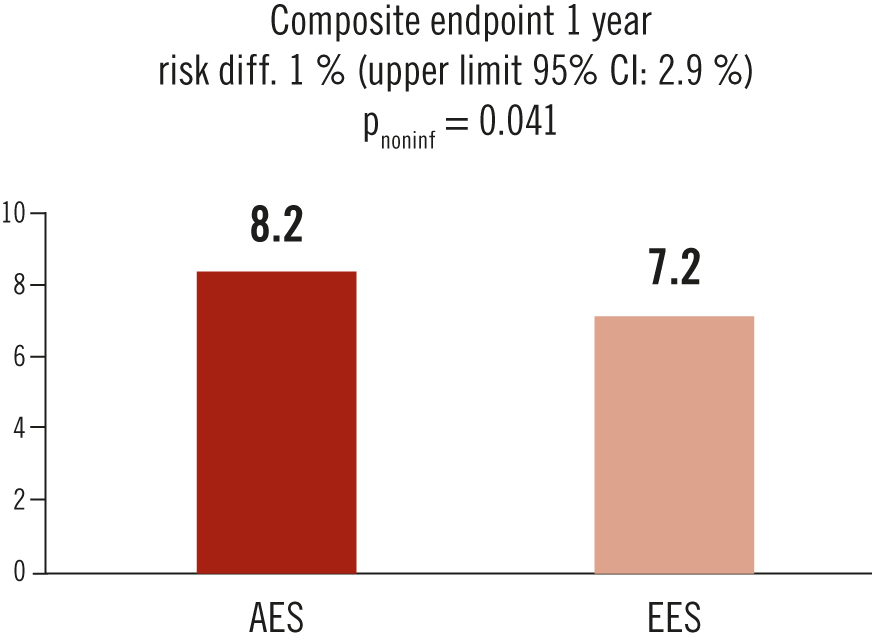
composite of cardiovascular death, MI, and lesion revascularisation at 1 year follow-up
Conclusion
in all-comer patients undergoing PCI, polymer-free AES were non-inferior to biodegradable-polymer EES at 1 year follow-up
Piccolo et al. EuroIntervention. 2025;21:e58-72