Objective
to investigate the safety and efficacy of prasugrel-based dose de-escalation therapy (5 mg) compared to the conventional maintenance dose prasugrel (10 mg) after PCI in ACS
Study
open-label, multicentre, non-inferiority trial (margin 2.5%)
Population
Asian patients with ACS undergoing PCI. All patients 100 mg aspirin daily and 10 mg prasugrel until 1 month follow-up. After 1 month de-escalation group 5 mg prasugrel and control: 10 mg prasugrel
Endpoints
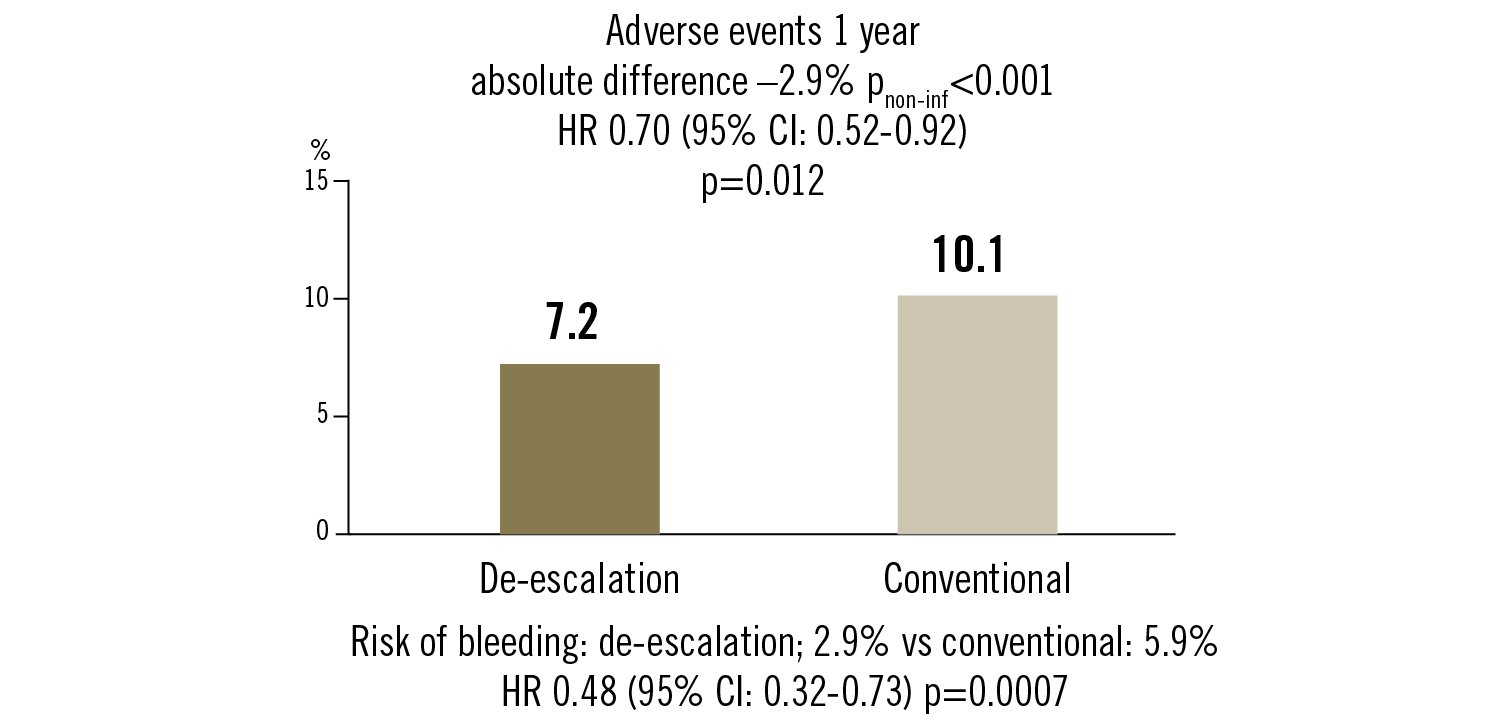
adverse events: all-cause death, non-fatal MI, stent thrombosis, repeat revascularisation, stroke, bleeding (BARC >grade 2) at 1 year
Conclusion
prasugrel-base dose de-escalation strategy (5 mg) after 1 month after PCI for ACS in Asian patients reduced adverse clinical events at 1 year compared to conventional treatment. This was mainly driven by reduced bleeding
Kim et al. Lancet. 2020;396:1079-89