Objective
to evaluate the safety and effectiveness of the PASCAL system compared to MitraClip in patients with significant symptomatic degenerative MR
Study
randomised controlled trial
Population
patients with 3+ or 4+ degenerative MR at prohibitive surgical risk
Endpoints
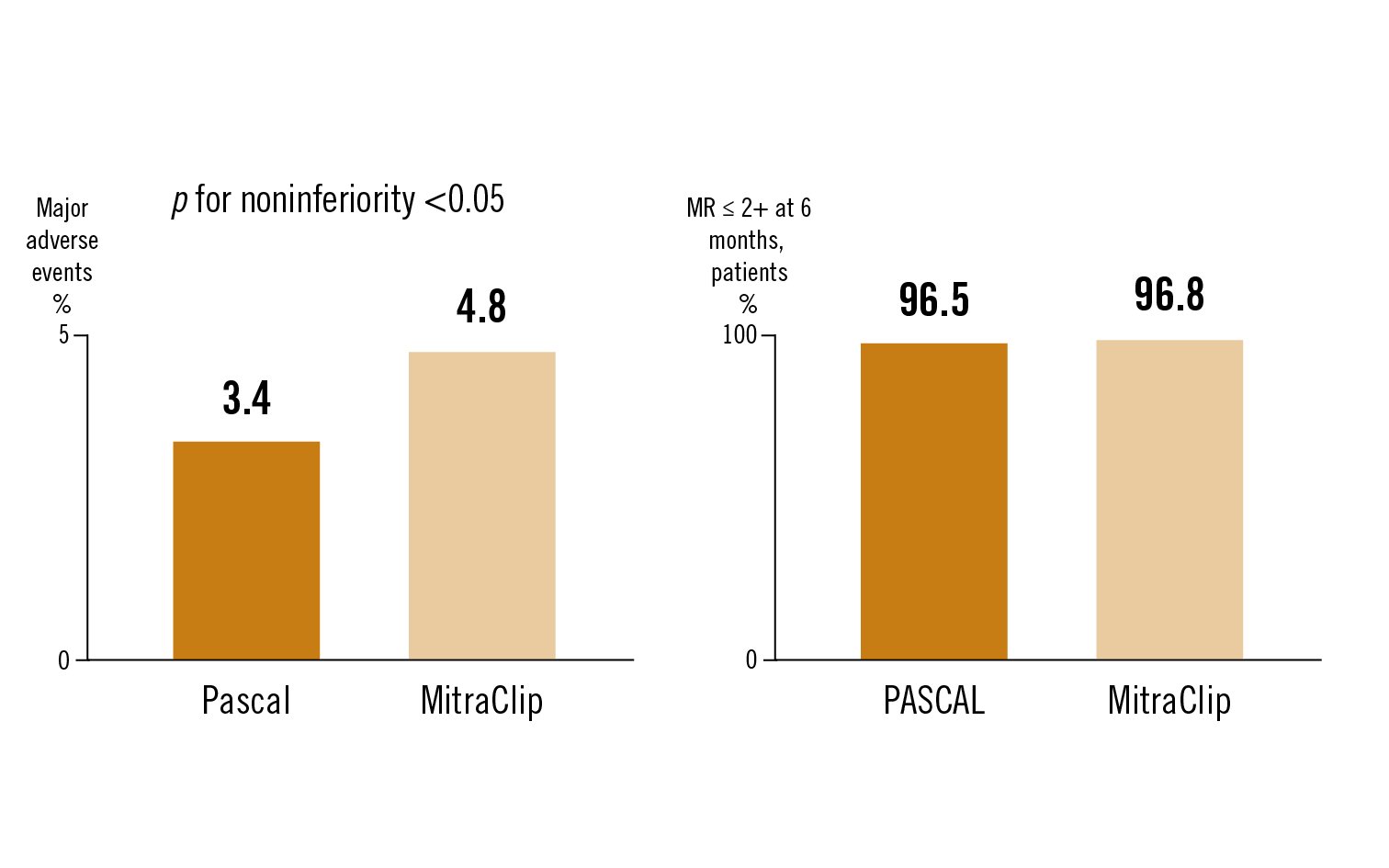
the primary safety endpoint was a composite major adverse event (MAE) rate at 30 days. The primary effectiveness endpoint was the proportion of patients with MR ⤠2+ at 6 months
Conclusion
the CLASP IID Trial demonstrated safety and effectiveness of the PASCAL system and met non-inferiority endpoints in comparison to MitraClip at 6 month follow-up
Scott Lim et al. JACC Cardiovasc Interv.2022; 15:2523-36