Objective
To evaluate the safety and efficacy of a self-expanding supraannular valve or a balloon-expandable valve in patients with severe asymptomatic aortic stenosis and an aortic-valve annulus area of 430 mm2 or less
Study
Prospective, postmarket, randomised trial
Population
Patients with severe asymptomatic aortic stenosis and an aortic-valve annulus area of 430 mm2 or less
Endpoints
A composite of death, disabling stroke, or rehospitalization for heart failure (tested for noninferiority) and a composite end point measuring bioprosthetic-valve dysfunction (tested for superiority).
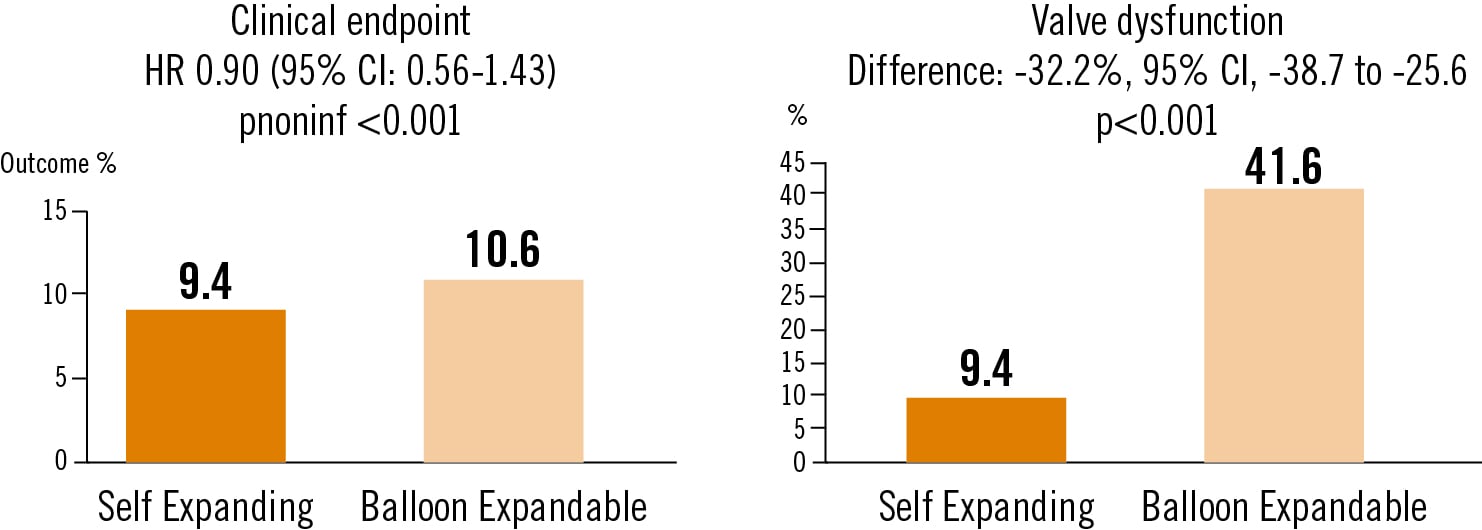
Conclusion
Among patients with severe aortic stenosis and small aortic annuli who were undergoing TAVR, clinical outcomes after implantation of a self-expanding supraannular valve were noninferior to those with a balloon-expandable valve, and the self-expanding valve was superior to the balloon-expandable valve with respect to bioprosthetic-valve dysfunction through 12 months.
Herrmann et al. NEJM 2024 DOI: 10.1056/NEJMoa2312573