Objective
to investigate the clinical outcomes of implantation of a sirolimus-bioabsorbable polymer-coated stent (MiStent) versus everolimus-eluting durable polymer stent (Xience)
Study
single-blind, multicentre, phase 3 non-inferiority randomised trial (phase 3 study; margin of 4.0%)
Population
all-comers
Endpoints
composite of cardiac death, target-vessel MI or clinically indicated T-L revascularisation at 1-year
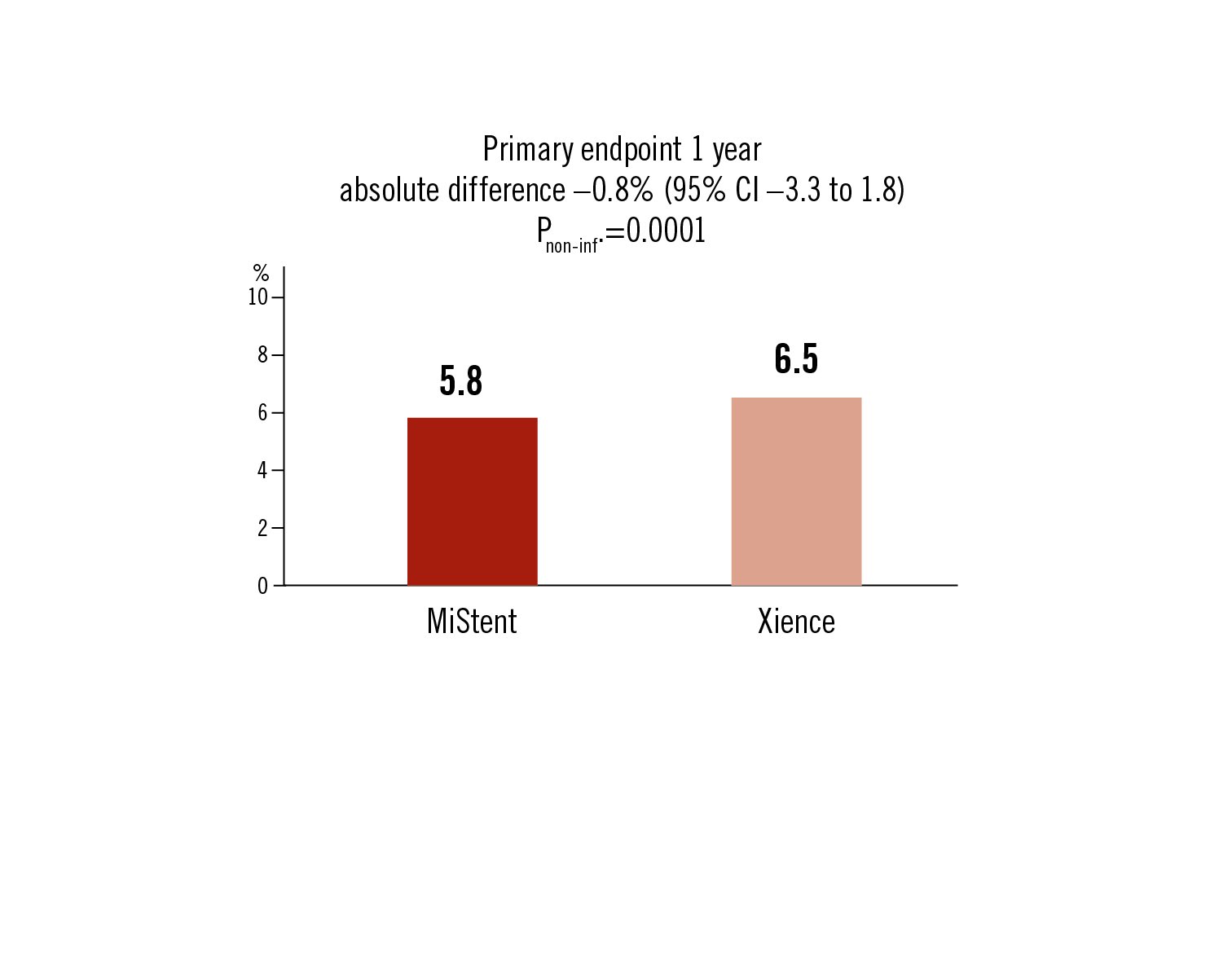
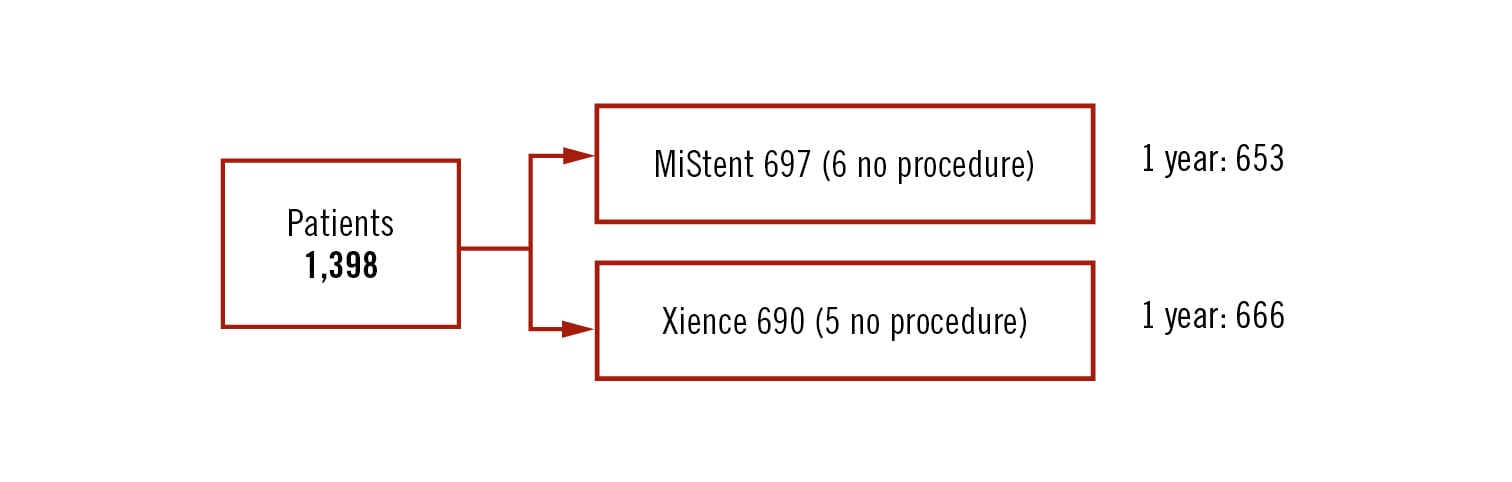
Conclusion
the sirolimus-eluting bioabsorbable polymer stent was non-inferior to the everolimus-eluting durable polymer stent during 1 year follow-up
de Winter et al. Lancet. 2018;391:431-40