Objective
to evaluate outcomes of the evolute low risk trial, where patients were randomised to TAVR with a self-expanding, supra-annular valve, or surgery, at 3 years
Study
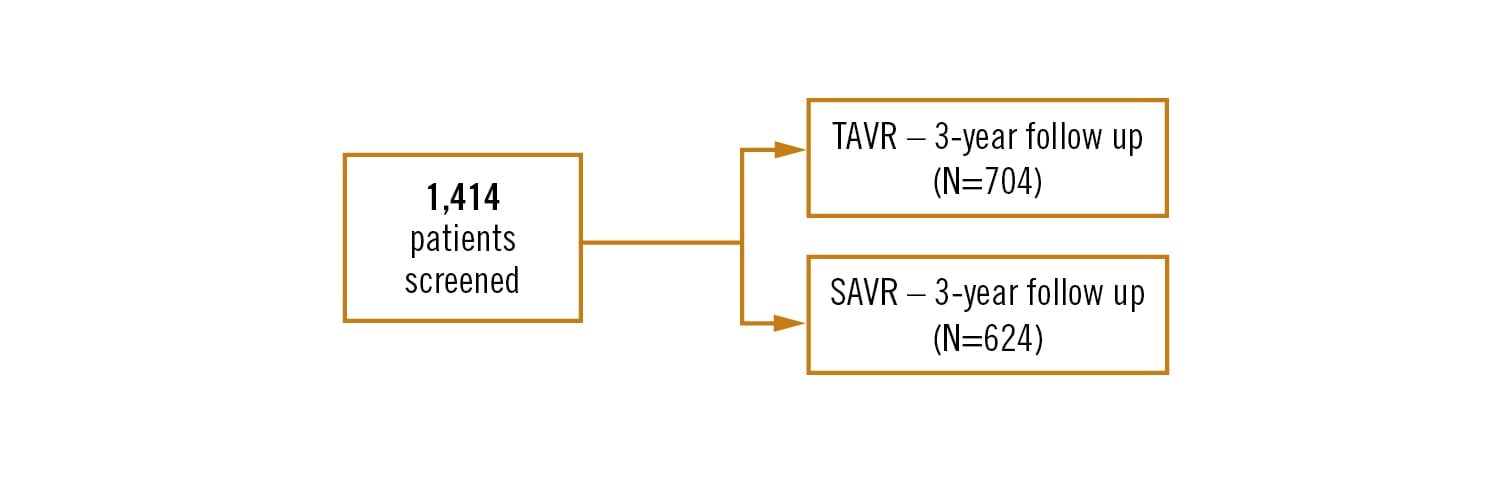
prospective, multinational, open label, randomised clinical trial
Population
patients with severe aortic stenosis who had a 30-day surgical mortality risk of <3% per local heart team assessment, confirmed by a national screening committee
Endpoints
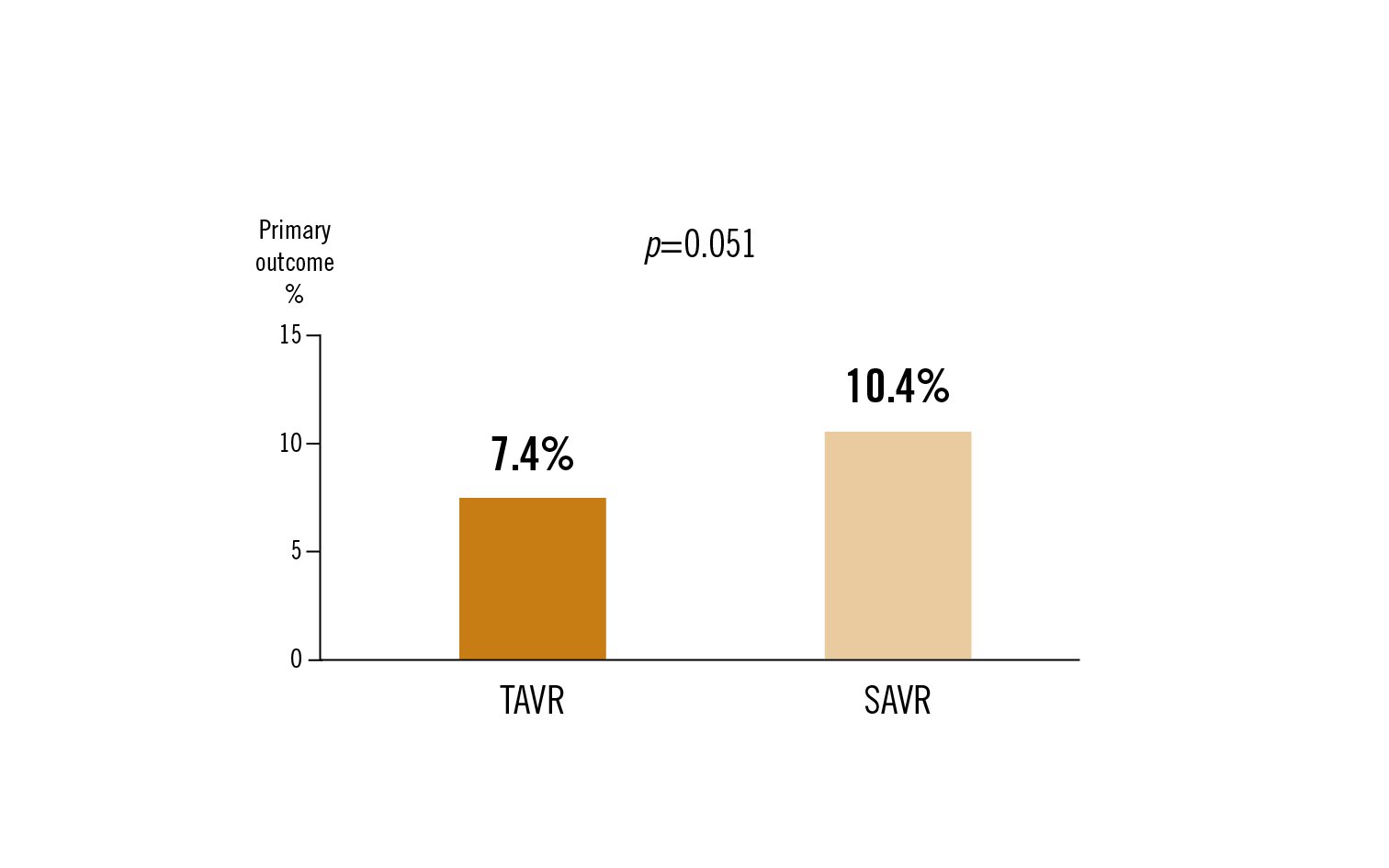
all-cause mortality or disabling stroke at 3 years
Conclusion
at 3 years, the difference between treatment arms for all-cause mortality or disabling stroke remained broadly consistent over time, with a trend towards decreased events in the TAVR group, although no statistically significant difference
Forrest et al NEJM. 2023