Objective
to determine whether switching to ticagrelor (90 mg twice daily) after 3 months DAPT (ticagrelor + aspirin) reduces adverse events compared to ticagrelor- based 12 month DAPT. Aspirin was discontinued after 3 months in patients with ticagrelor based- 12 month DAPT
Study
multicentre randomised trial
Population
patients with ACS and successful ORSIRO stent implantation
Endpoints
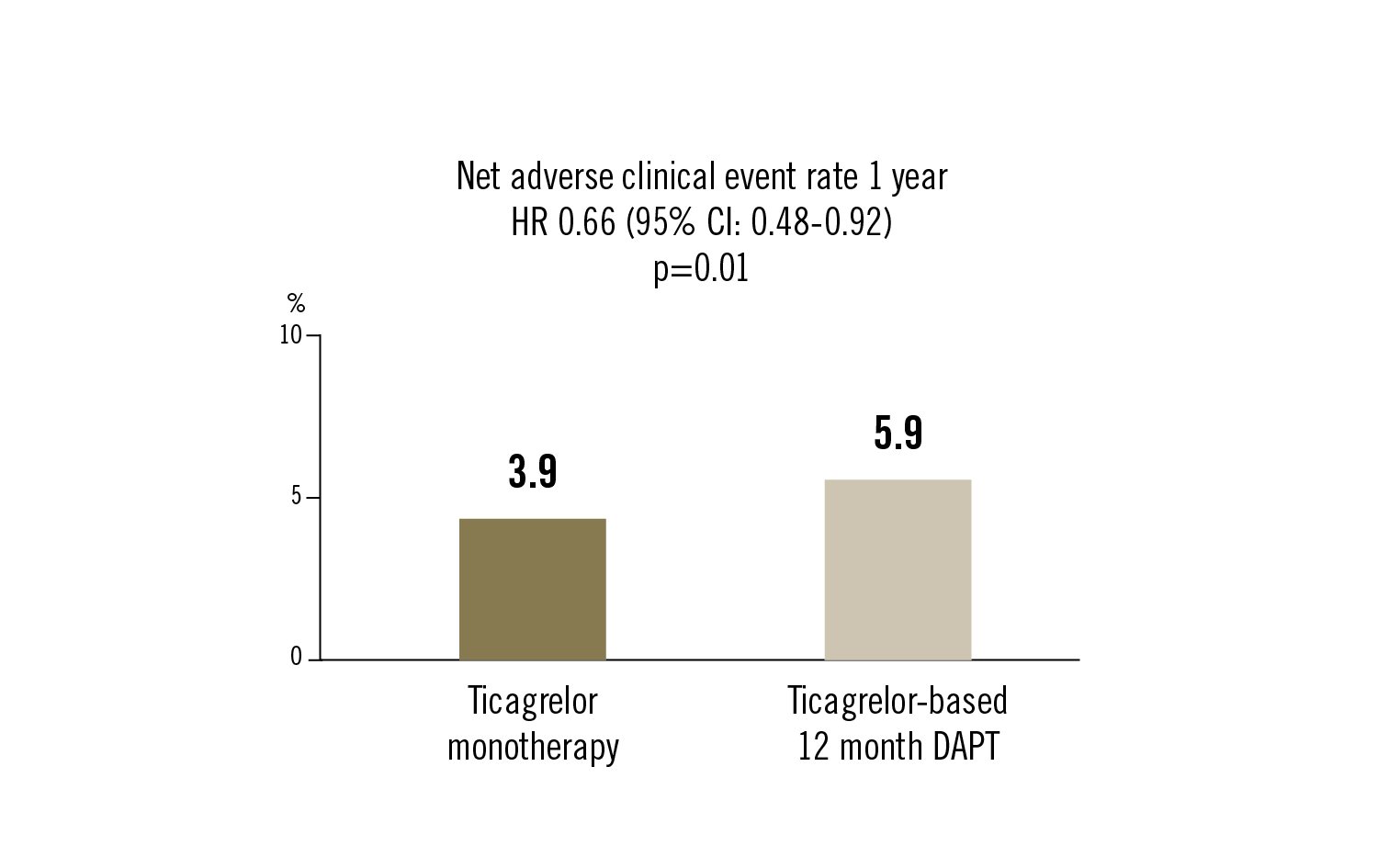
1-year net adverse clinical event defined as composite of major bleeding and adverse cardiac and cerebrovascular events (death, MI, stent thrombosis, stroke or target-vessel revascularisation)
Conclusion
ticagrelor monotherapy after 3 months of DAPT compared to ticagrelor-based 12 month DAPT was associated with a modest reduction in net adverse clinical events at 1 year
Kim et al. JAMA. 2020;323:2407-16