Objective
to report the 1-year clinical outcomes of ultra-thin, biodegradable polymer sirolimus-eluting stent (BP-SES) compared to thin, durablepolymer, everolimus-eluting stent (DP-EES) for treatment of coronary artery lesions
Study
investigator-initiated, prospective, open-label, multicentre noninferiority randomised trial (margin 3.3%)
Population
all-comers
Endpoints
target lesion failure: composite cardiac death, MI and clinically driven revascularisation at 1 year
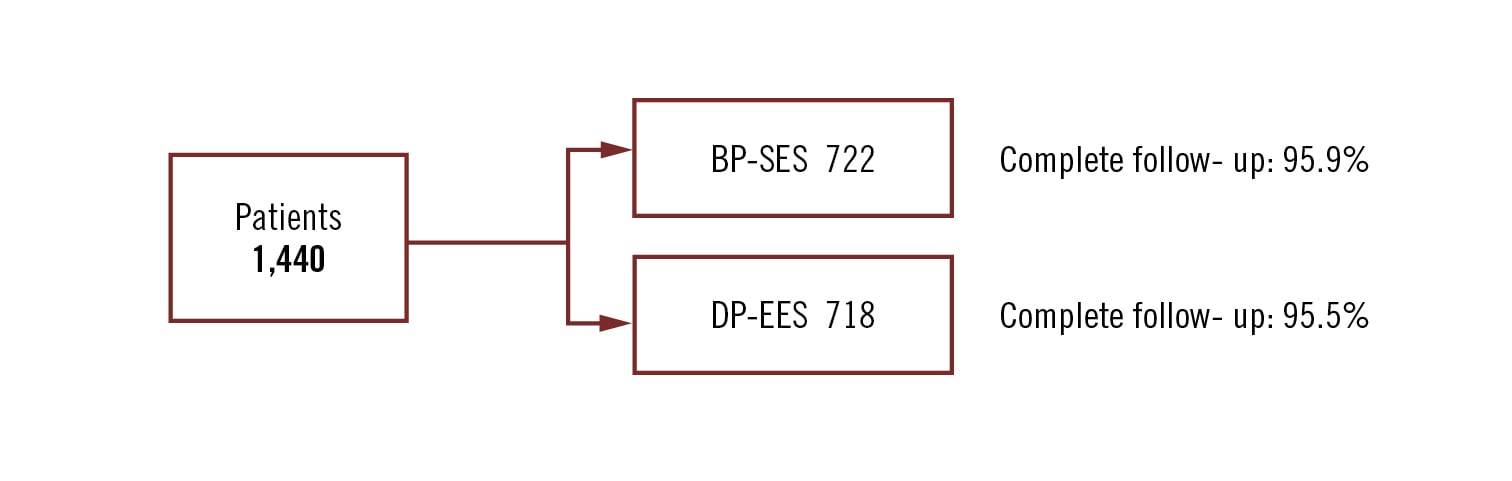
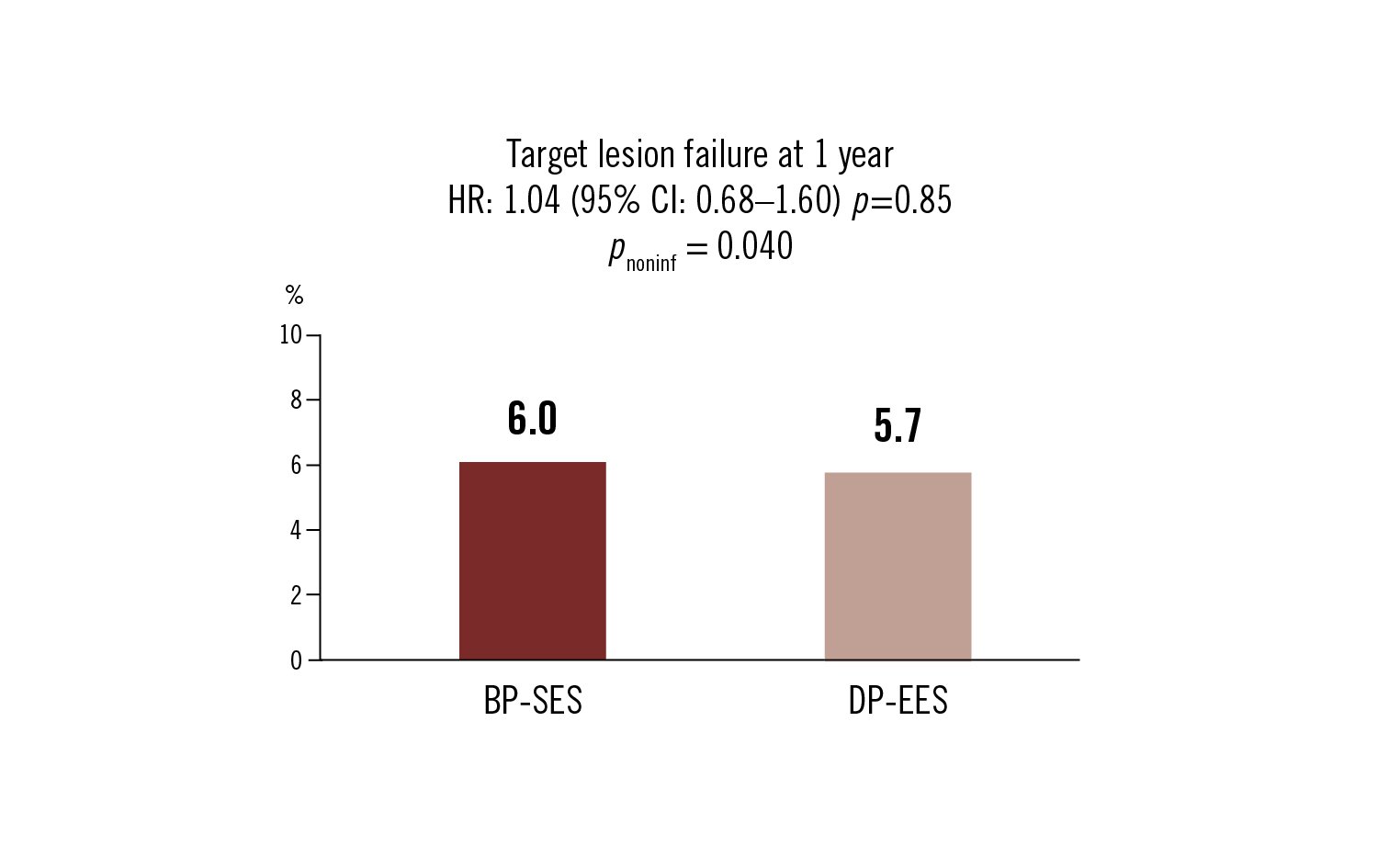
Conclusion
BP-SES was non-inferior to DP-EES at 1 year
Nakamura et al. J Am Coll Cardiol Interv. 2022;15:1324-34